Metabolic challenges in CAR-T cell therapy
CAR-T cell therapy has already shown great success in treating advanced hematologic malignancies. However, its efficacy in solid tumors has been unsatisfactory, in part due to low tumor-specific antigens, heterogeneous expression of tumor-associated antigens, insufficient persistence of T cells, dysregulation of T cell metabolism, and immunosuppressive tumor microenvironment (TME) [1]. Metabolic reprogramming or interference with metabolic requirements within tumors leads to decreased antitumor activity of T cells [2], and improving the metabolic adaptation of T cells can greatly enhance their persistence and antitumor activity.
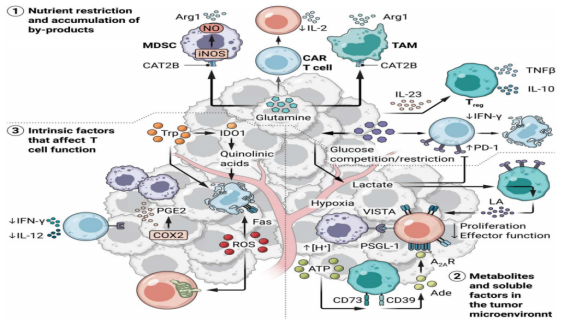
Image credit: Sci Immunol. 2023 Apr 14; 8(82)
Metabolic reprogramming of CAR-T cells improves tumor control
Metabolic adaptation of cancer cells and other tumor infiltrating cells
Cancer cells are metabolically active, consuming large amounts of glucose, amino acids and fatty acids from the environment to meet their nutritional needs. The rapid growth of the tumor results in nutrient deficiency and hypoxic TME. Aerobic glycolysis, the catabolism of glucose into lactic acid even in the presence of oxygen, is primarily employed by cancer cells. This adaptation supports energy requirements while preserving metabolic intermediates that can serve as carbon sources for lipid, amino acid, and nucleotide synthesis to support large-scale growth and proliferation. Interestingly, inhibitory tumor-infiltrating immune cells, including M2-like tumor-associated macrophages (TAMs), myeloid inhibitory cells (MDSCs), and regulatory T cells (Tregs), are well adapted to acidic, nutrient-deprived, and hypoxic Tmes [3]. For example, lactic acid promotes TAM differentiation into an M2-like phenotype characterized by elevated expression of arginase 1 (Arg1) and the accumulation of MDSCs in TME, rather than macrophages or dendritic cells [4]. Lactic acid and fatty acids can also be used as fuels to support the inhibition of Tregs [5]. The therapeutic potential of targeting these metabolic adaptations by inhibiting uptake of high-demand nutrients or specific metabolic pathways by tumors and tumor-infiltrating cells is currently being investigated.
Metabolic stress causes T cells to collapse
T cell subpopulations (including naive (TN), effector (effector), memory (memory), stem cell memory (TSCM), and depleted T cells) depend on different metabolic characteristics for survival and anti-tumor immune function. Intracellular and extracellular metabolites within TME can drive immunosuppression and coordinate T cell failure. For example, glycolysis can support the production of effector cytokines by regulating specific metabolites, such as interferon-gamma [6]. Lactic acid in TME can also inhibit T cell proliferation and effector function through various mechanisms [7.8]. Some amino acids, including arginine, tryptophan, and glutamine, are strongly associated with tumor development and the fate of T cells. However, the deficiency of these amino acids in TME can lead to dysfunction of CAR T cells and tumor-infiltrating lymphocytes (TILs). In addition to nutritional limitations, some metabolites and soluble factors produced in TME, including immunosuppressive mediators, reactive oxygen species (ROS), and prostaglandin E2 (PGE2), can directly induce T cell dysfunction [9]. Recombinant hypoxia response may regulate T cell differentiation and maintain the persistence and antitumor response of CAR T cells. Understanding metabolic regulation within TME provides a critical foundation for designing metabolic programs of CAR-T cells to maximize their persistence and functional capacity within tumors.

Image credit: Sci Immunol. 2023 Apr 14; 8(82)
Metabolic reprogramming of CAR-T cells improves tumor control
Strategies for promoting memory signatures, resisting immunosuppressive signals and depletion programs in CAR-T cells are being intensively studied. Metabolic reprogramming is a key step in the differentiation and survival of memory T cells, and metabolic insufficiency can drive the failure program of T cells during chronic viral infection and tumor invasion [10]. Therefore, in CAR-T cells, modulating metabolic fitness to promote memory T cell differentiation is considered a promising approach to improve persistence, overcome hostile TME, and maintain CAR-T cell function against chronic antigenic stimulation [9]. Studies have found that CAR-T cell persistence and effector function can be maintained in the following ways:
(1) Selection of CAR signaling motifs - enhancing the persistence of T cells: co-stimulatory signals provided by co-stimulatory domains in CAR can not only enhance the effect function, but also affect the metabolic program of CAR T cells. In preclinical models of solid tumors and malignant hematologic diseases, Myd88-CD40ζ (MC) CAR T cells showed strong proliferative ability, resistance to exhaustion procedures, and superior tumor control ability compared with CD28ζ and 4-1BBζ CAR T cells [11]. MC CAR-T cells showed a poorly differentiated state and were enriched in FOXM1 and MYB target genes [11]. FOXM1 has been shown to be a positive regulator of glycolysis and oxidative phosphorylation (OXPHOS) in multiple myeloma and hepatocellular carcinoma. In addition, co-stimulatory signals from OX40 also promote T cell memory phenotypes. The second-generation CAR-T cells expressing the OX40 co-stimulatory signal domain showed strong proliferation ability [12]. How co-stimulatory domains in CAR-T cells can be modified to optimize metabolic reprogramming to maximize persistence and antitumor activity in vivo remains to be clarified.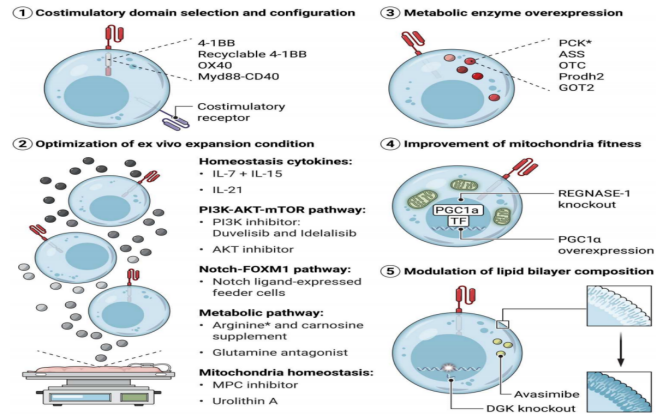
Image credit: Sci Immunol. 2023 Apr 14; 8 (82).
In summary, improving the persistence of CAR T cells and overcoming resistance to TME metabolic stress are key challenges for CAR T cell therapy. The fate of T cells infiltrating TME and binding to tumor targets is closely related to the metabolic reprogramming that occurs within the tumor. Understanding the dysregulation of T cell metabolism in TME can identify key targets for reinvigorating T cell antitumor activity. In preclinical studies, strategies that use metabolic pathways to promote memory T cell formation or reverse the effect-function inhibition of nutrient-restricted TME can effectively improve the efficacy of CAR-T cells in tumor control.
References:
[1]. J. Wagner, E. Wickman, C. DeRenzo, S. Gottschalk, CAR T cell therapy for solid tumors: Bright future or dark reality? Mol. Ther. 28, 2320–2339 (2020).
[2]. F. Franco, A. Jaccard, P. Romero, Y. R. Yu, P. C. Ho, Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2, 1001–1012 (2020).
[3]. F. Li, M. C. Simon, Cancer cells don't live alone: Metabolic communication within tumor microenvironments. Dev. Cell 54, 183–195 (2020).
[4]. J. L. Zhao, Y. C. Ye, C. C. Gao, L. Wang, K. X. Ren, R.Jiang, S.J. Hu, S. Q. Liang,J. Bai,J. L. Liang,P. F. Ma, Y. Y. Hu, B. C. Li, Y. Z. Nie, Y. Chen, X. F. Li, W. Zhang, H. Han, H. Y. Qin, Notchmediated lactate metabolism regulates MDSC development through the Hes1/MCT2/cJun axis. Cell Rep. 38, 110451 (2022).
[5]. H. Wang, F. Franco, Y. C. Tsui, X. Xie, M. P. Trefny, R. Zappasodi, S. R. Mohmood,
J. Fernández-García, C. H. Tsai, I. Schulze, F. Picard, E. Meylan, R. Silverstein, I. Goldberg,S. M. Fendt, J. D. Wolchok, T. Merghoub, C. Jandus, A. Zippelius, P. C. Ho, CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat.Immunol. 21, 298–308 (2020).
[6]. M. Peng, N. Yin, S. Chhangawala, K. Xu, C. S. Leslie, M. O. Li, Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354,
481–484 (2016).
[7]. W. J. Quinn III, J. Jiao, T. T. Slaa, J. Stadanlick, Z. Wang, L. Wang, T. Akimova, A. Angelin,P. M. Schäfer, M. D. Cully, C. Perry, P. K. Kopinski, L. Guo, I. A. Blair, L. R. Ghanem,M. S. Leibowitz, W. W. Hancock, E. K. Moon, M. H. Levine, E. B. Eruslanov, D. C. Wallace,J. A. Baur, U. H. Beier, Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep.33, 108500 (2020).
[8]. H. Rostamian, M. Khakpoor-Koosheh, L. Jafarzadeh, E. Masoumi, K. Fallah-Mehrjardi,M. J. Tavassolifar, J. M. Pawelek, H. R. Mirzaei, J. Hadjati, Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer 22, 39 (2022).
[9]. Peng JJ, Wang L, Li Z, Ku CL, Ho PC. Metabolic challenges and interventions in CAR T cell therapy. Sci Immunol. 2023 Apr 14;8(82):eabq3016. doi:10. 1126/sciimmunol.abq3016. Epub 2023 Apr 14. PMID: 37058548.
[10]. B. Bengsch, A. L. Johnson, M. Kurachi, P. M. Odorizzi, K. E. Pauken, J. Attanasio, E. Stelekati,L. M. McLane, M. A. Paley, G. M. Delgoffe, E. J. Wherry, Bioenergetic insufficiencies due to metabolic alterationsregulated by the inhibitory receptor PD-1 are an early driver of CD8+T cell exhaustion. Immunity 45, 358–373 (2016).
[11]. B. Prinzing, P. Schreiner, M. Bell, Y. Fan, G. Krenciute, S. Gottschalk, MyD88/CD40 signaling retains CAR T cells in a less differentiated state. JCI Insight 5, e136093 (2020).
[12]. J. Tan, Y. Jia, M. Zhou, C. Fu, I. J. Tuhin, J. Ye, M. A. Monty, N. Xu, L. Kang, M. Li, J. Shao,X. Fang, H. Zhu, L. Yan, C. Qu, S. Xue, Z. Jin, S. Chen, H. Huang, Y. Xu, J. Chen, M. Miao,X. Tang, C. Li, Z. Yan, D. Wu, L. Yu, Chimeric antigen receptors containing the OX40 signalling domain enhance the persistence of T cells even under repeated stimulation with multiple myeloma target cells. J. Hematol. Oncol. 15, 39 (2022).
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@sz-cell.com
(1) Selection of CAR signaling motifs - enhancing the persistence of T cells: co-stimulatory signals provided by co-stimulatory domains in CAR can not only enhance the effect function, but also affect the metabolic program of CAR T cells. In preclinical models of solid tumors and malignant hematologic diseases, Myd88-CD40ζ (MC) CAR T cells showed strong proliferative ability, resistance to exhaustion procedures, and superior tumor control ability compared with CD28ζ and 4-1BBζ CAR T cells [11]. MC CAR-T cells showed a poorly differentiated state and were enriched in FOXM1 and MYB target genes [11]. FOXM1 has been shown to be a positive regulator of glycolysis and oxidative phosphorylation (OXPHOS) in multiple myeloma and hepatocellular carcinoma. In addition, co-stimulatory signals from OX40 also promote T cell memory phenotypes. The second-generation CAR-T cells expressing the OX40 co-stimulatory signal domain showed strong proliferation ability [12]. How co-stimulatory domains in CAR-T cells can be modified to optimize metabolic reprogramming to maximize persistence and antitumor activity in vivo remains to be clarified.
(2)Optimization of metabolic strategies during CAR-T cell generation: Optimization of in vitro expansion protocols can induce metabolic reprogramming and support the formation of TSCM and TCM. For example, IL-2 promotes glycolysis to support rapid proliferation and is commonly used in CAR-T cell cultures. However, inhibition of glycolysis in CAR-T cells has been shown to maintain a poorly differentiated state, extend persistence in vivo, and enhance anti-tumor activity [13]. Therefore, regulating the concentration and timing of IL-2 or replacing IL-2 with other cytokines during CAR-T cell expansion may be an effective way to increase the ratio of TSCM-like cells to TCM-like cells. Other cytokines, including IL-7, IL-15, and IL-21, have been found to promote oxidative phosphorylation and contribute to the formation of memory phenotypes. In preclinical studies and Phase I clinical trials, including in patients with neuroblastoma, melanoma, sarcoma, and osteosarcoma, IL-7 and IL-15 have been found to minimize CAR-T cell depletion and provide excellent antitumor activity [13]. In addition to cytokine signaling, optimizing the levels of other nutrients during amplification also affects CAR T cell infusion products.

Image credit: Sci Immunol. 2023 Apr 14; 8 (82).
In summary, improving the persistence of CAR T cells and overcoming resistance to TME metabolic stress are key challenges for CAR T cell therapy. The fate of T cells infiltrating TME and binding to tumor targets is closely related to the metabolic reprogramming that occurs within the tumor. Understanding the dysregulation of T cell metabolism in TME can identify key targets for reinvigorating T cell antitumor activity. In preclinical studies, strategies that use metabolic pathways to promote memory T cell formation or reverse the effect-function inhibition of nutrient-restricted TME can effectively improve the efficacy of CAR-T cells in tumor control.
References:
[1]. J. Wagner, E. Wickman, C. DeRenzo, S. Gottschalk, CAR T cell therapy for solid tumors: Bright future or dark reality? Mol. Ther. 28, 2320–2339 (2020).
[2]. F. Franco, A. Jaccard, P. Romero, Y. R. Yu, P. C. Ho, Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2, 1001–1012 (2020).
[3]. F. Li, M. C. Simon, Cancer cells don't live alone: Metabolic communication within tumor microenvironments. Dev. Cell 54, 183–195 (2020).
[4]. J. L. Zhao, Y. C. Ye, C. C. Gao, L. Wang, K. X. Ren, R.Jiang, S.J. Hu, S. Q. Liang,J. Bai,J. L. Liang,P. F. Ma, Y. Y. Hu, B. C. Li, Y. Z. Nie, Y. Chen, X. F. Li, W. Zhang, H. Han, H. Y. Qin, Notchmediated lactate metabolism regulates MDSC development through the Hes1/MCT2/cJun axis. Cell Rep. 38, 110451 (2022).
[5]. H. Wang, F. Franco, Y. C. Tsui, X. Xie, M. P. Trefny, R. Zappasodi, S. R. Mohmood,
J. Fernández-García, C. H. Tsai, I. Schulze, F. Picard, E. Meylan, R. Silverstein, I. Goldberg,S. M. Fendt, J. D. Wolchok, T. Merghoub, C. Jandus, A. Zippelius, P. C. Ho, CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat.Immunol. 21, 298–308 (2020).
[6]. M. Peng, N. Yin, S. Chhangawala, K. Xu, C. S. Leslie, M. O. Li, Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354,
481–484 (2016).
[7]. W. J. Quinn III, J. Jiao, T. T. Slaa, J. Stadanlick, Z. Wang, L. Wang, T. Akimova, A. Angelin,P. M. Schäfer, M. D. Cully, C. Perry, P. K. Kopinski, L. Guo, I. A. Blair, L. R. Ghanem,M. S. Leibowitz, W. W. Hancock, E. K. Moon, M. H. Levine, E. B. Eruslanov, D. C. Wallace,J. A. Baur, U. H. Beier, Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep.33, 108500 (2020).
[8]. H. Rostamian, M. Khakpoor-Koosheh, L. Jafarzadeh, E. Masoumi, K. Fallah-Mehrjardi,M. J. Tavassolifar, J. M. Pawelek, H. R. Mirzaei, J. Hadjati, Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer 22, 39 (2022).
[9]. Peng JJ, Wang L, Li Z, Ku CL, Ho PC. Metabolic challenges and interventions in CAR T cell therapy. Sci Immunol. 2023 Apr 14;8(82):eabq3016. doi:10. 1126/sciimmunol.abq3016. Epub 2023 Apr 14. PMID: 37058548.
[10]. B. Bengsch, A. L. Johnson, M. Kurachi, P. M. Odorizzi, K. E. Pauken, J. Attanasio, E. Stelekati,L. M. McLane, M. A. Paley, G. M. Delgoffe, E. J. Wherry, Bioenergetic insufficiencies due to metabolic alterationsregulated by the inhibitory receptor PD-1 are an early driver of CD8+T cell exhaustion. Immunity 45, 358–373 (2016).
[11]. B. Prinzing, P. Schreiner, M. Bell, Y. Fan, G. Krenciute, S. Gottschalk, MyD88/CD40 signaling retains CAR T cells in a less differentiated state. JCI Insight 5, e136093 (2020).
[12]. J. Tan, Y. Jia, M. Zhou, C. Fu, I. J. Tuhin, J. Ye, M. A. Monty, N. Xu, L. Kang, M. Li, J. Shao,X. Fang, H. Zhu, L. Yan, C. Qu, S. Xue, Z. Jin, S. Chen, H. Huang, Y. Xu, J. Chen, M. Miao,X. Tang, C. Li, Z. Yan, D. Wu, L. Yu, Chimeric antigen receptors containing the OX40 signalling domain enhance the persistence of T cells even under repeated stimulation with multiple myeloma target cells. J. Hematol. Oncol. 15, 39 (2022).
[13]. J. D. Chan, J. Lai, C. Y. Slaney, A. Kallies, P. A. Beavis, P. K. Darcy, Cellular networks controlling T cell persistence in adoptive cell therapy. Nat. Rev. Immunol. 21, 769–784 (2021).
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@sz-cell.com


