In-depth assessment of the development process of CRS - providing the best intervention window and r
Chimeric antigen receptor-modified T cell (CAR-T) therapy is currently a powerful approach to combat hematological malignancies, but cytokine release syndrome (CRS) remains the most common complication of CAR-T therapy. Even though the management of CAR-T toxicity has been greatly improved, CRS remains one of the major safety issues. In theory, CRS is an indispensable inflammatory response in CAR-T therapy, and appropriate CRS is thought to help reduce cancer cells. However, excessive CRS can cause damage to important organs, putting patients at risk.
Severe CRS (≥ grade 3) was reported in 46% of patients with B-cell acute lymphoblastic leukemia (ALL) treated with CD19 CAR-T and 41% of patients with multiple myeloma (MM) treated with BCMA CAR-T. Therefore, it is necessary to gain a deeper understanding of the mechanism of CRS in order to properly manage severe immune reactions.
Recently, the team of Academician Chen Saijuan from Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine published a paper titled "Neutrophil activation and clonal CAR-T re-expansion underpinning cytokine release syndrome during ciltacabtagene autoleucel therapy in multiple myeloma" in Nature Communications. The paper longitudinally monitored the serum cytokine levels and circulating immune cell transcriptomes of 26 r/r MM patients treated with Cilta-cel (a dual-epitope CAR-T product targeting BCMA antigen developed by Johnson & Johnson/Nanjing Legend) to understand the immune dynamics of CRS. The severity of CRS can be accurately described and potentially predicted by the temporal cytokine secretion characteristics. The results of the study showed that there is a latent period with obvious immune changes before obvious CRS, which provides the best window and potential target for CRS treatment intervention. CAR-T re-expansion requires close clinical attention and laboratory research to reduce the risk of mortality.
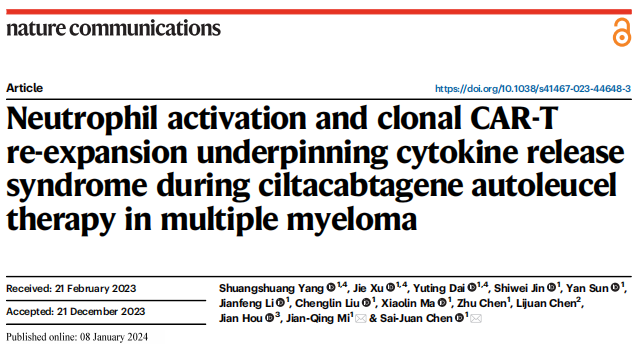
CRS events were observed in all patients within 30 days of Cilta-cel administration, with a median time to onset of 6 days (range, 1 to 10 days). In 65.4% of cases, fever was the earliest event, with an average onset time of 6 days. In the remaining 34.6% of patients, hypotension, hypoxemia, or organ dysfunction appeared earlier than fever in the CRS outbreak. 34.6% of patients had grade 1 to 2 CRS, and 65.4% of patients had grade 3 or above. CRS grade was assessed based on clinical symptoms, and changes in CRS grade were consistent with the circulating kinetics of Cilta-cel. As CRS progresses, the CD8:CD4 ratio in CAR-T gradually increases and reaches a peak on days 16 to 20. After summarizing the individual treatment responses of CRS in terms of symptoms, grade, management, and outcomes, it was found that there was no significant difference in clinical outcomes between patients with severe and mild CRS, indicating that clinical prognosis is independent of CRS grade.
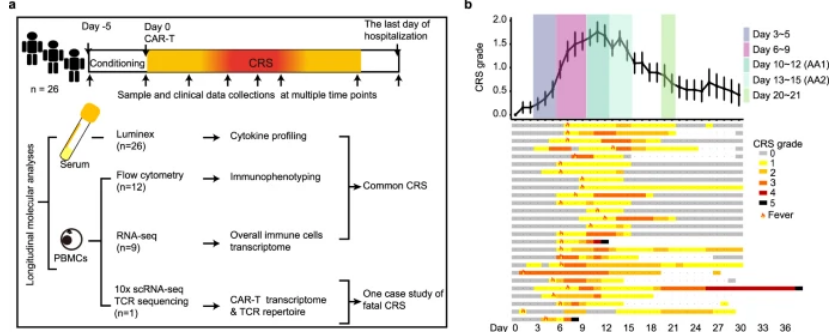
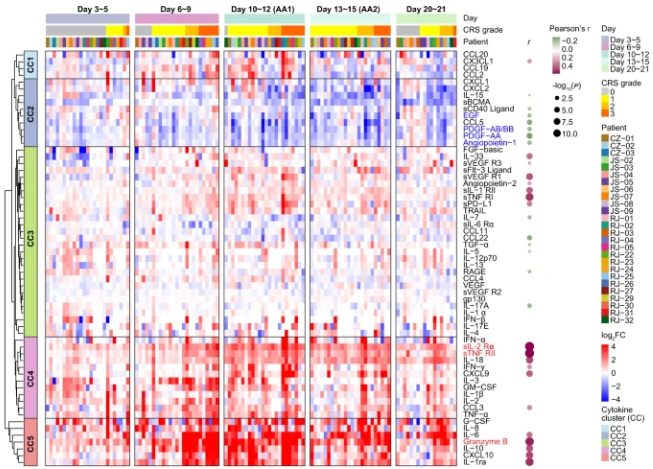
In order to gain a deeper understanding of the development of CRS, the researchers divided the patient's clinical inflammatory response within 21 days into five periods, including baseline (before infusion), incubation period (days 3 to 5), and fever period (days 6 to 9). ), acute exacerbation period (days 10 to 15, AA) and resolution period (days 20 to 21). Serum data from the above time periods were uated to create an overall picture of the cytokine profile. Compared with baseline, inflammatory molecules were significantly upregulated in the later stages of fever and reached higher levels during the acute exacerbation phase, with IL-6, granzyme B, IL-10, G-CSF, and CXCL10 soaring 50 to 100 times at the peak. , indicating a strong inflammatory response in the period from day 6 to day 15. Through unsupervised clustering, 5 cytokine clusters (CCs) were identified, among which CC3, CC4, and CC5 were positively correlated with the average CRS grade, while CC2 was negatively correlated. Notably, among the cytokines that were positively correlated with CRS severity, sIL-2Rα, sTNF RII (all belong to CC4) and granzyme B (all belong to CC5) showed the strongest correlation.
Additionally, to better understand the timing of the immune cell activation cascade, the researchers also performed a gene set enrichment analysis (GSEA) to compare each time point to baseline. It was found that neutrophils reacted most strongly on days 3 to 5 (latency period), while the immune responses of macrophages and T cells started from the latency period and responded strongly during the fever period on days 6 to 9, indicating that neutrophils are the precursors to the inflammatory response, and macrophages
and T lymphocytes are more involved in the subsequent cytokine cascade. With the response of immune cells, the key inflammatory signaling pathways TNF, IL-6/JAK-STAT3, JAK/STAT, IL-2/STAT5 and complement/coagulation cascade were significantly up-regulated, and the gene set score was also obtained. reached similar conclusions. These data can predict potential targets and the appropriate time to use signaling blockers.
In summary, this study deeply uated the physiological process and timing of the development of CRS associated with CAR-T therapy and deepened the understanding of the systemic toxicity of cellular immunotherapy. The step-by-step process of CRS induced by Cilta-cel describes the dynamic changes of the signal transduction-cytokine-presentation axis. On the one hand, the inflammatory signaling pathway was activated 3 to 5 days before the cytokine storm, indicating that doctors need to monitor and care closely as early as the 3rd to 5th day to reduce the uncontrollable toxic side effects caused by delayed intervention; on the other hand, the overactivation of IL-2/STAT5, IL-6/JAK-STAT3, and TNF-α signal transduction indicates a potential target for drug administration, and the corresponding antagonists can be used to block severe cytokine storms.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com
Severe CRS (≥ grade 3) was reported in 46% of patients with B-cell acute lymphoblastic leukemia (ALL) treated with CD19 CAR-T and 41% of patients with multiple myeloma (MM) treated with BCMA CAR-T. Therefore, it is necessary to gain a deeper understanding of the mechanism of CRS in order to properly manage severe immune reactions.
Recently, the team of Academician Chen Saijuan from Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine published a paper titled "Neutrophil activation and clonal CAR-T re-expansion underpinning cytokine release syndrome during ciltacabtagene autoleucel therapy in multiple myeloma" in Nature Communications. The paper longitudinally monitored the serum cytokine levels and circulating immune cell transcriptomes of 26 r/r MM patients treated with Cilta-cel (a dual-epitope CAR-T product targeting BCMA antigen developed by Johnson & Johnson/Nanjing Legend) to understand the immune dynamics of CRS. The severity of CRS can be accurately described and potentially predicted by the temporal cytokine secretion characteristics. The results of the study showed that there is a latent period with obvious immune changes before obvious CRS, which provides the best window and potential target for CRS treatment intervention. CAR-T re-expansion requires close clinical attention and laboratory research to reduce the risk of mortality.

CRS events were observed in all patients within 30 days of Cilta-cel administration, with a median time to onset of 6 days (range, 1 to 10 days). In 65.4% of cases, fever was the earliest event, with an average onset time of 6 days. In the remaining 34.6% of patients, hypotension, hypoxemia, or organ dysfunction appeared earlier than fever in the CRS outbreak. 34.6% of patients had grade 1 to 2 CRS, and 65.4% of patients had grade 3 or above. CRS grade was assessed based on clinical symptoms, and changes in CRS grade were consistent with the circulating kinetics of Cilta-cel. As CRS progresses, the CD8:CD4 ratio in CAR-T gradually increases and reaches a peak on days 16 to 20. After summarizing the individual treatment responses of CRS in terms of symptoms, grade, management, and outcomes, it was found that there was no significant difference in clinical outcomes between patients with severe and mild CRS, indicating that clinical prognosis is independent of CRS grade.

In order to gain a deeper understanding of the development of CRS, the researchers divided the patient's clinical inflammatory response within 21 days into five periods, including baseline (before infusion), incubation period (days 3 to 5), and fever period (days 6 to 9). ), acute exacerbation period (days 10 to 15, AA) and resolution period (days 20 to 21). Serum data from the above time periods were uated to create an overall picture of the cytokine profile. Compared with baseline, inflammatory molecules were significantly upregulated in the later stages of fever and reached higher levels during the acute exacerbation phase, with IL-6, granzyme B, IL-10, G-CSF, and CXCL10 soaring 50 to 100 times at the peak. , indicating a strong inflammatory response in the period from day 6 to day 15. Through unsupervised clustering, 5 cytokine clusters (CCs) were identified, among which CC3, CC4, and CC5 were positively correlated with the average CRS grade, while CC2 was negatively correlated. Notably, among the cytokines that were positively correlated with CRS severity, sIL-2Rα, sTNF RII (all belong to CC4) and granzyme B (all belong to CC5) showed the strongest correlation.

Additionally, to better understand the timing of the immune cell activation cascade, the researchers also performed a gene set enrichment analysis (GSEA) to compare each time point to baseline. It was found that neutrophils reacted most strongly on days 3 to 5 (latency period), while the immune responses of macrophages and T cells started from the latency period and responded strongly during the fever period on days 6 to 9, indicating that neutrophils are the precursors to the inflammatory response, and macrophages
and T lymphocytes are more involved in the subsequent cytokine cascade. With the response of immune cells, the key inflammatory signaling pathways TNF, IL-6/JAK-STAT3, JAK/STAT, IL-2/STAT5 and complement/coagulation cascade were significantly up-regulated, and the gene set score was also obtained. reached similar conclusions. These data can predict potential targets and the appropriate time to use signaling blockers.
In summary, this study deeply uated the physiological process and timing of the development of CRS associated with CAR-T therapy and deepened the understanding of the systemic toxicity of cellular immunotherapy. The step-by-step process of CRS induced by Cilta-cel describes the dynamic changes of the signal transduction-cytokine-presentation axis. On the one hand, the inflammatory signaling pathway was activated 3 to 5 days before the cytokine storm, indicating that doctors need to monitor and care closely as early as the 3rd to 5th day to reduce the uncontrollable toxic side effects caused by delayed intervention; on the other hand, the overactivation of IL-2/STAT5, IL-6/JAK-STAT3, and TNF-α signal transduction indicates a potential target for drug administration, and the corresponding antagonists can be used to block severe cytokine storms.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com
Previous:Nothing



