Comparison of CAR-NK cells derived from adult peripheral blood and umbilical cord blood
Among hematological cancers, acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) are the most common leukemias in children and the elderly, respectively. Some patients do not respond to chemotherapy and adjuvant immunotherapy is necessary, such as chimeric antigen receptor (CAR) therapy, which is one of the newest and more effective treatments for these cancers and B-cell lymphomas. However, CAR-T cell therapy still carries some risks, including cytokine release syndrome (CRS) and neurotoxicity. Therefore, some researchers are committed to developing other CAR therapeutic vectors. NK cells from different sources are one of the promising vehicles for CAR therapy because they do not cause graft-versus-host disease (GvHD) in allogeneic therapy and they rapidly attack cancer without prior sensitization. cell.
Researchers from Spain published a research article titled "Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells" in Scientific reports. This article mainly studies the effects of NK cells from adult peripheral blood (AB) and umbilical cord blood (CB) on different target cells to determine the best source of CAR therapy.
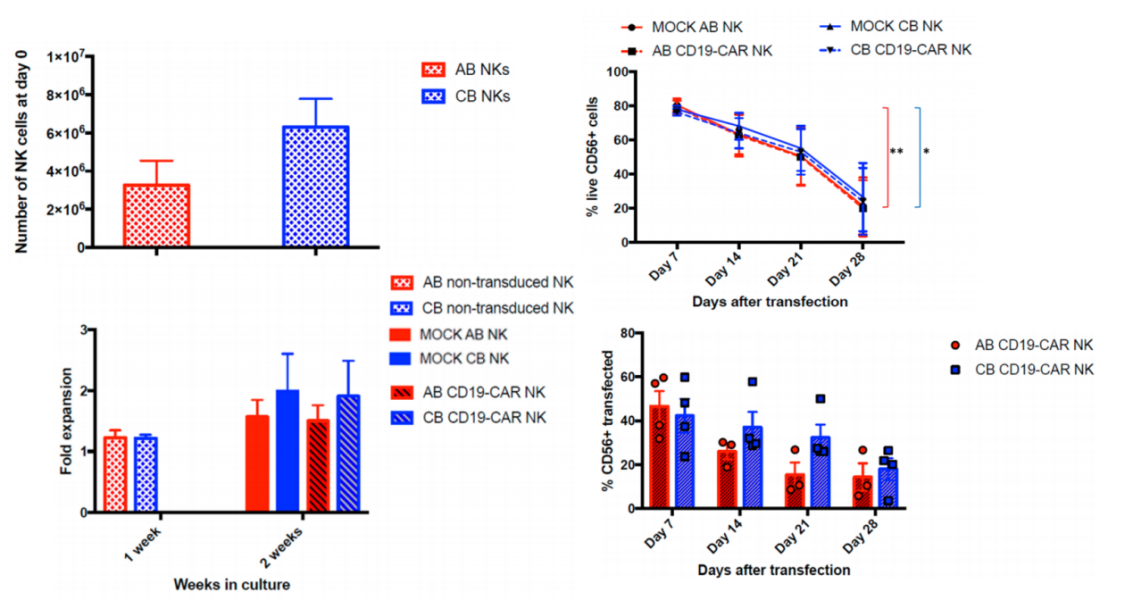
1. In vitro expansion and CD19 CAR transduction of NK cells from two sources:
NK cells were isolated from AB or CB PBMC by negative selection, and the results showed that more NK cells were isolated from CB than from AB. A slightly higher fold expansion was observed in CB NK cells after two weeks, but the expansion fold was also lower, with only 2-3-fold expansion at 2 weeks. After viral transduction, the cell survival rate decreased over time. By day 28, the survival rate of CAR-NK derived from both cells dropped significantly to about 20%. In terms of transduction efficiency, the average transduction efficiency of AB CAR-NK was 47.46%, while the transduction efficiency of CB CAR-NK was 46.8%. No statistical difference was found between the transduction efficiency of AB and CB NK cells. Regardless of whether it is CAR-NK prepared from NK cells derived from AB or CB , the viability and purity of Shenzhen Cell Valley are above 90%, the positive rate of CAR transduction is as high as 60~80%, and the amplification factor can reach tens of thousands of times.
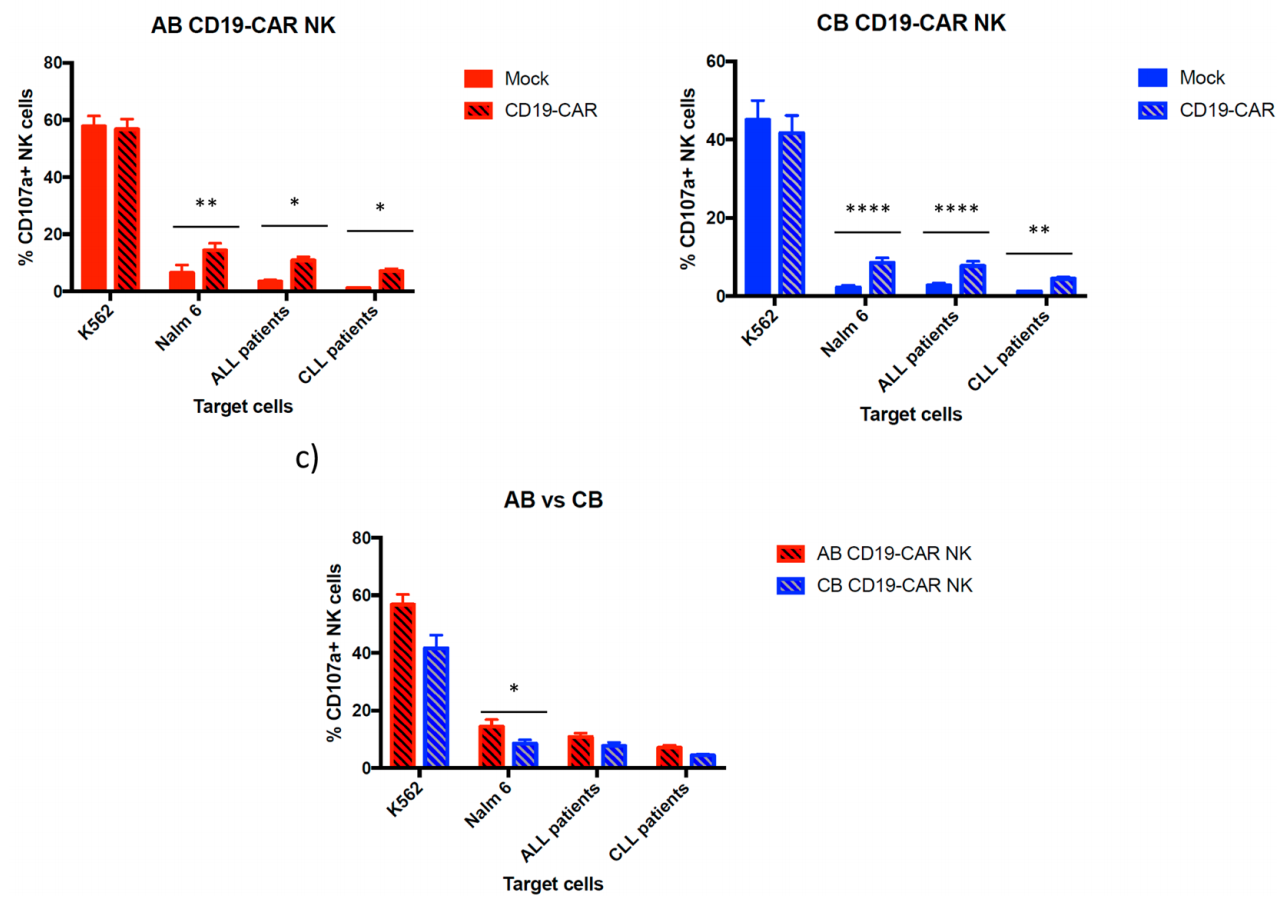
2. CD19-CAR-NK cells from two sources cause degranulation after encountering cells expressing CD19.
There is a direct correlation between the surface expression level of CD107a and the cytotoxic activity and cytokine secretion of NK cells. This study compared the degranulation ability of CD19 CAR-NK cells and non-transduced NK cells on CD19-expressing cells by measuring the expression of CD107a on the cell surface . Experimental results showed that AB and CB NK cells transduced or not transduced with CD19 CAR showed similar degranulation activity in the presence of K562. AB CD19 CAR-NK cells showed higher degranulation activity than untransduced AB NK cells in CD19-positive cells such as Nalm-6, ALL and CLL primary patient tumor cells . CB CD19 CAR-NK cells showed higher degranulation activity than untransduced CB NK cells. The expression of CD107a in AB CD19 CAR-NK cells is slightly higher than that in CB CD19 CAR-NK cells.
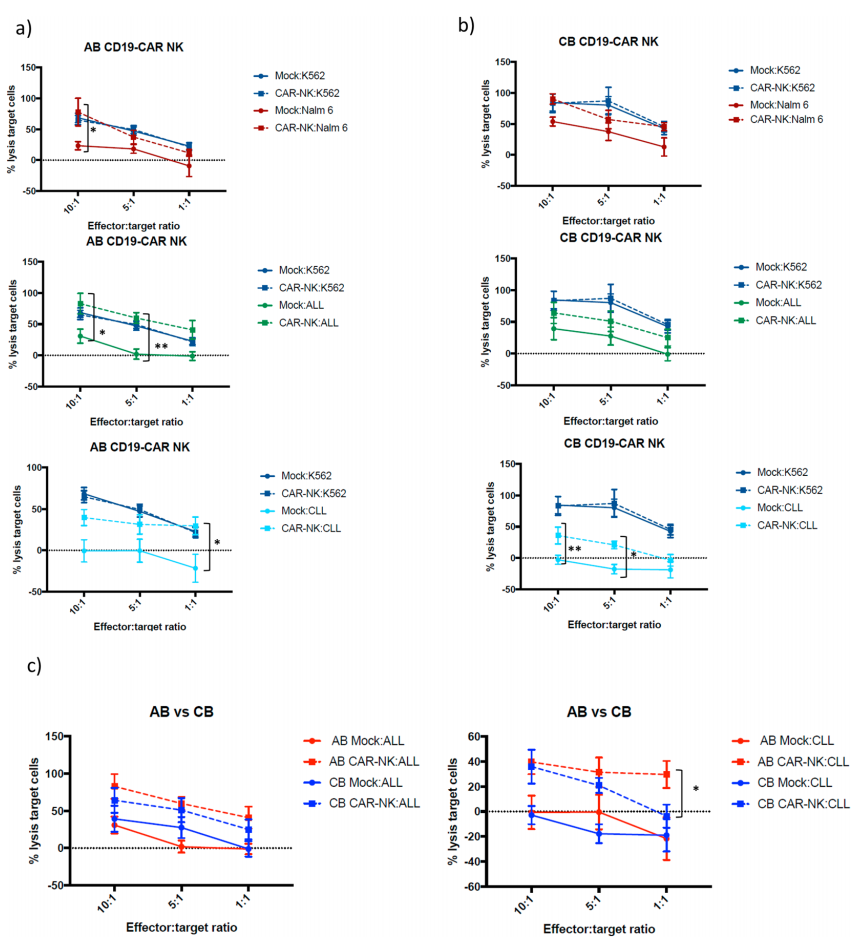
3. CD19-CAR-transduced NK cells kill CD19-expressing cells more effectively
This study examined the cytotoxic activity of non-transduced NK cells and CD19 CAR-NK cells from two sources under different effector-to-target ratios. AB and CB CAR-NK cells lysed CD19-expressing target cells better than untransduced NK cells. AB CD19 CAR-NK cells are significantly better than CB CD19-CAR NK cells in killing Nalm-6 at 10:1 ratio, killing ALL at 10:1 and 5:1 ratios, and killing CLL cells at 1:1 ratio. . On the other hand, the ability of CB CD19-CAR NK cells to lyse CLL cells was significantly enhanced at the 10:1 and 5:1 ratios. Summary : Regardless of whether it is tumor cells from primary patients with ALL or CLL, the killing ability of CD19-CAR NK cells derived from AB is significantly better than that derived from CB.
In summary , AB CAR-NK cells perform slightly better in killing target cells expressing CD19 , while CB NK cells have a relatively stable cell number per unit and can improve in vitro expansion, killing activity and survival rate through different interleukin stimulation. The above experiments show that CAR-NK cells from both sources have their advantages, and both can be used as alternatives and safer candidate cells for CAR therapy. Both are suitable for future clinical application of CAR-NK in the treatment of hematological diseases.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com
Researchers from Spain published a research article titled "Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells" in Scientific reports. This article mainly studies the effects of NK cells from adult peripheral blood (AB) and umbilical cord blood (CB) on different target cells to determine the best source of CAR therapy.

1. In vitro expansion and CD19 CAR transduction of NK cells from two sources:
NK cells were isolated from AB or CB PBMC by negative selection, and the results showed that more NK cells were isolated from CB than from AB. A slightly higher fold expansion was observed in CB NK cells after two weeks, but the expansion fold was also lower, with only 2-3-fold expansion at 2 weeks. After viral transduction, the cell survival rate decreased over time. By day 28, the survival rate of CAR-NK derived from both cells dropped significantly to about 20%. In terms of transduction efficiency, the average transduction efficiency of AB CAR-NK was 47.46%, while the transduction efficiency of CB CAR-NK was 46.8%. No statistical difference was found between the transduction efficiency of AB and CB NK cells. Regardless of whether it is CAR-NK prepared from NK cells derived from AB or CB , the viability and purity of Shenzhen Cell Valley are above 90%, the positive rate of CAR transduction is as high as 60~80%, and the amplification factor can reach tens of thousands of times.

2. CD19-CAR-NK cells from two sources cause degranulation after encountering cells expressing CD19.
There is a direct correlation between the surface expression level of CD107a and the cytotoxic activity and cytokine secretion of NK cells. This study compared the degranulation ability of CD19 CAR-NK cells and non-transduced NK cells on CD19-expressing cells by measuring the expression of CD107a on the cell surface . Experimental results showed that AB and CB NK cells transduced or not transduced with CD19 CAR showed similar degranulation activity in the presence of K562. AB CD19 CAR-NK cells showed higher degranulation activity than untransduced AB NK cells in CD19-positive cells such as Nalm-6, ALL and CLL primary patient tumor cells . CB CD19 CAR-NK cells showed higher degranulation activity than untransduced CB NK cells. The expression of CD107a in AB CD19 CAR-NK cells is slightly higher than that in CB CD19 CAR-NK cells.

3. CD19-CAR-transduced NK cells kill CD19-expressing cells more effectively
This study examined the cytotoxic activity of non-transduced NK cells and CD19 CAR-NK cells from two sources under different effector-to-target ratios. AB and CB CAR-NK cells lysed CD19-expressing target cells better than untransduced NK cells. AB CD19 CAR-NK cells are significantly better than CB CD19-CAR NK cells in killing Nalm-6 at 10:1 ratio, killing ALL at 10:1 and 5:1 ratios, and killing CLL cells at 1:1 ratio. . On the other hand, the ability of CB CD19-CAR NK cells to lyse CLL cells was significantly enhanced at the 10:1 and 5:1 ratios. Summary : Regardless of whether it is tumor cells from primary patients with ALL or CLL, the killing ability of CD19-CAR NK cells derived from AB is significantly better than that derived from CB.

In summary , AB CAR-NK cells perform slightly better in killing target cells expressing CD19 , while CB NK cells have a relatively stable cell number per unit and can improve in vitro expansion, killing activity and survival rate through different interleukin stimulation. The above experiments show that CAR-NK cells from both sources have their advantages, and both can be used as alternatives and safer candidate cells for CAR therapy. Both are suitable for future clinical application of CAR-NK in the treatment of hematological diseases.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com



