Introduction to the whole process of CAR-T cell clinical treatment: Taking lymphoma as an example
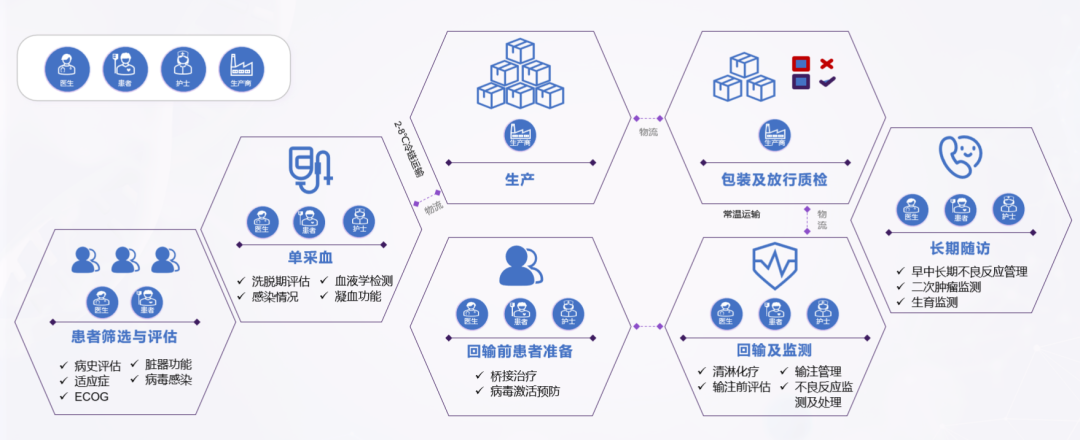
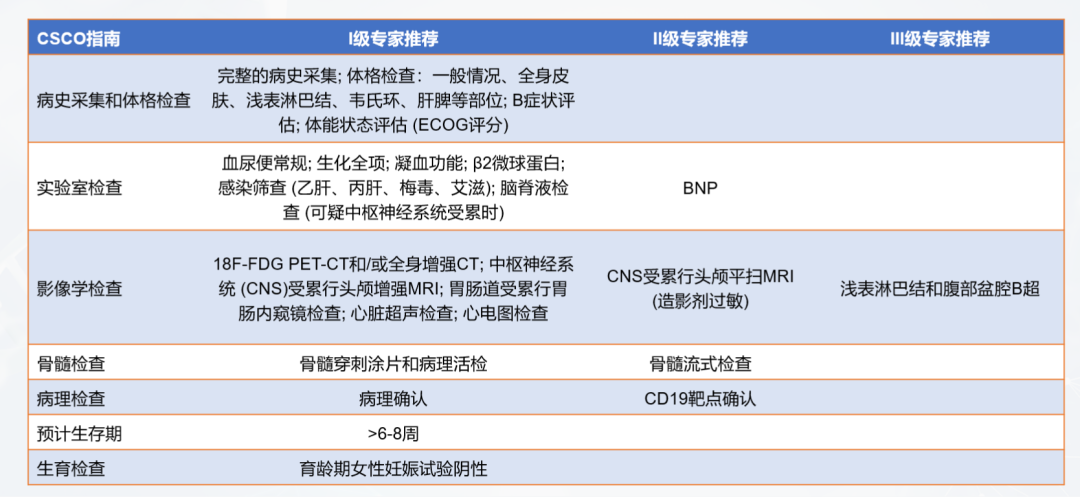
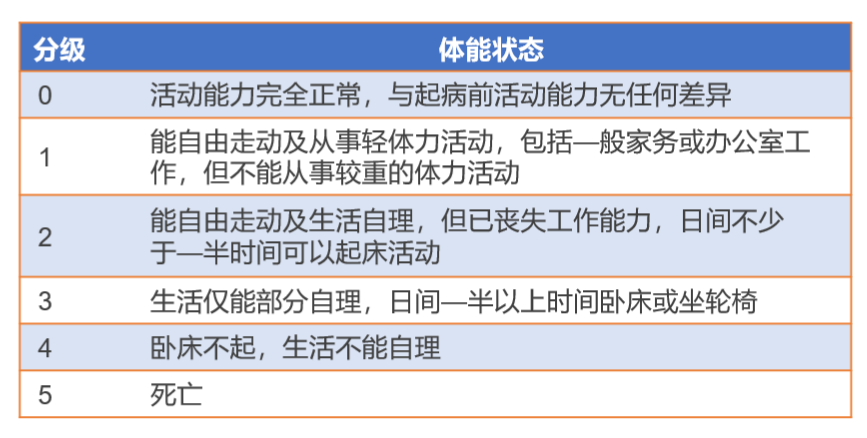
How do cancer patients know whether their condition is suitable for CAR-T treatment? There are many clinical criteria. For example, the Guidelines for the Management of Infections Related to CAR-T Cell Therapy for Malignant Hematological Tumors and Immunotargeted Therapy of the Chinese Society of Clinical Oncology (CSCO) as shown in Figure 2, patients need to collect medical history and undergo physical examinations, laboratory tests, and imaging examinations under the guidance of a doctor. The doctor will assess whether the patient meets the treatment conditions based on the examination results and physical indicators.
Before collecting peripheral blood mononuclear cells from patients, it is still necessary to check the patient's physical condition and blood cell status. This is to determine whether the patient's own T cell status can meet the needs of autologous CAR-T cell preparation. If the activity is too poor, consider using allogeneic T cells from healthy people for preparation. There are many factors that may affect lymphocyte collection, which are mainly determined by the following biochemical substances. First, avoid lymphocyte toxic drugs before blood separation to prevent T cell collection failure, and avoid using immunosuppressants as much as possible to prevent reducing the vitality of the immune system. Granulocyte colony stimulating factor (G-CSF) induces stem cell mobilization, and stem cells in leukocyte separation products are at risk of malignant transformation during genetic modification by viral transduction. Therefore, G-CSF should be stopped before leukocyte separation. Corticosteroids can cause rapid consumption of lymphocytes in the blood circulation, and low to moderate doses of hormones may cause a slight decrease in lymphocytes. Ibrutinib can selectively inhibit Th2 responses and reduce the expression of PD-1, a marker of exhaustion in T cells, which may enhance the function of CAR-T cells. Therefore, Bruton's tyrosine kinase (BTK) inhibitors can be used before cell isolation, and studies on their simultaneous use with CAR-T cells are underway [4].
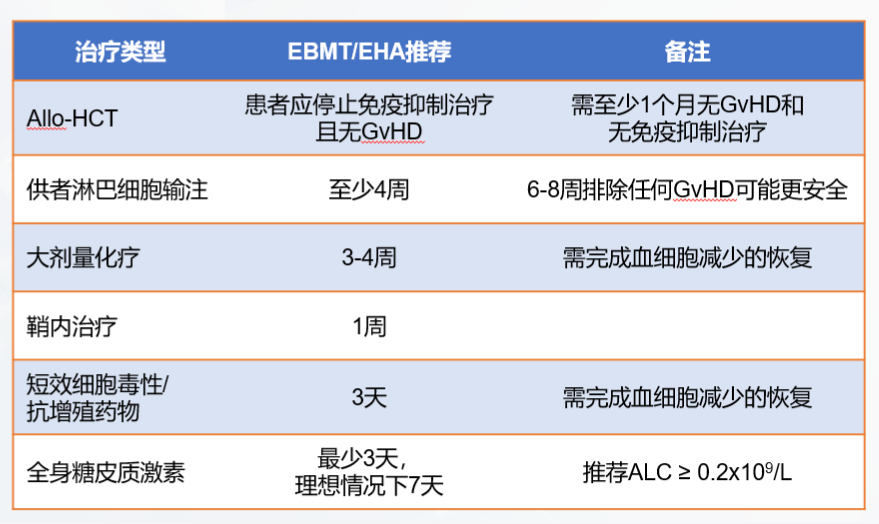
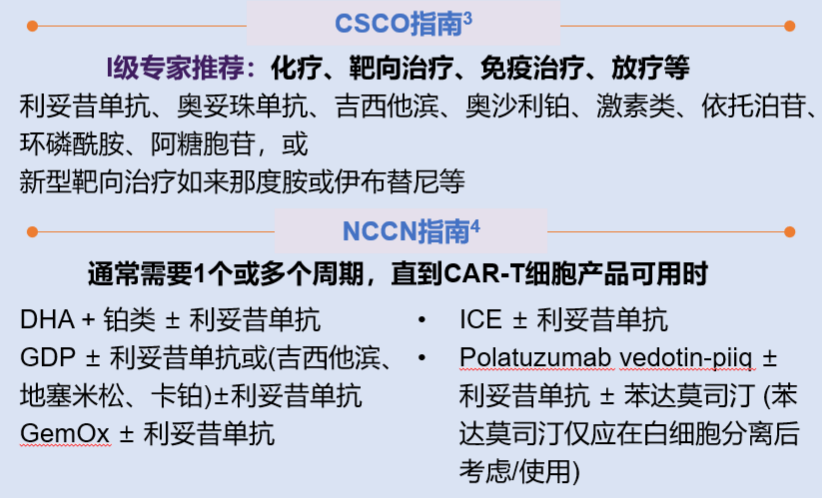
Bridging therapy may be given after mononuclear cell collection and before CAR-T cell infusion. Bridging therapy does not include lymphocyte depletion chemotherapy. The purpose is to prevent rapid disease progression, reduce tumor burden, and relieve tumor-related symptoms. If the expected turnover time of CAR-T cells is short, bridging therapy can be omitted if the disease is stable and the tumor burden is low. If during the preparation of CAR-T cells, the doctor determines that the patient's tumor progression may affect the cell infusion, bridging therapy may be considered. The specific treatment plan is shown in Figure 5.
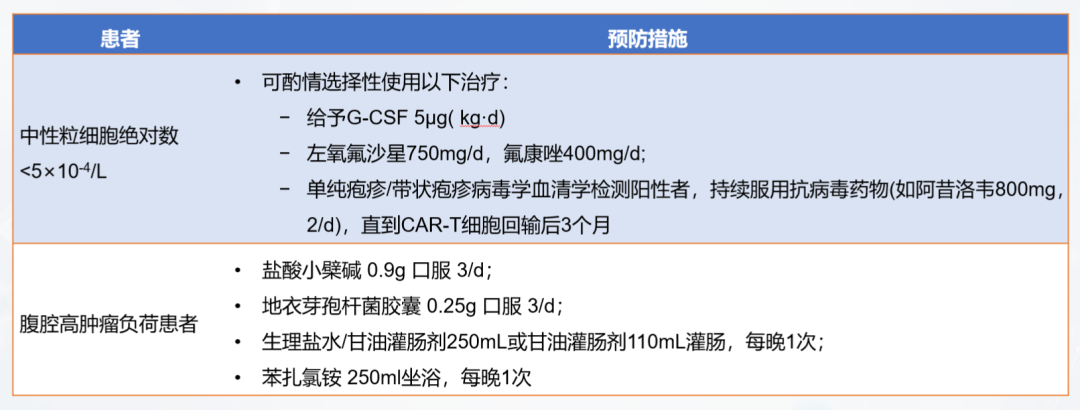
In order to remove normal lymphocytes in the patient's body, eliminate immunosuppressive factors, and create an immune microenvironment that is conducive to the expansion of CAR-T cells, lymphocyte clearance chemotherapy is required before CAR-T transfusion. The commonly used regimen for lymphocyte clearance chemotherapy is fludarabine + cyclophosphamide (FC regimen). After chemotherapy, the patient's immune level drops significantly. During this process, it is necessary to prevent the patient from infection. Common infection prevention and control measures are shown in Figure 6.
04 CAR-T cell reinfusion
Before CAR-T cell transfusion (before thawing of CAR-T cell products), the patient's clinical status needs to be comprehensively uated again, including vital signs (body temperature, blood pressure, blood oxygen saturation, heart rate), active infection, and organ function. Active infection and hypotension requiring vasopressor therapy are contraindications for CAR-T cell transfusion, and CAR-T cell transfusion needs to be delayed until the infection or hypotension is completely treated or controlled.

CAR-T cell transfusion is critical and requires many clinical conditions to be met, such as establishing central venous access, preferably using a double-lumen or triple-lumen catheter for intravenous infusion and possible vasopressors; ECG monitoring in the event of clinically severe arrhythmias, and additional monitoring based on clinical indications; according to standard institutional guidelines, it is recommended that patients with large tumor burdens and aggressive histology undergo tumor lysis prevention and monitoring; and seizure prevention starting on the day of CAR-T cell therapy infusion, which is known to cause CAR-T cell-related neurotoxicity, etc. [7].
Before CAR-T cell transfusion, check product information, reconstitute the product, adjust the infusion rate, and start transfusing cells. Generally, ensure that the time from the end of cell recovery to the full transfusion is controlled within 30 minutes. Record the start and end time of transfusion, monitor the patient's blood pressure, heart rate and other indicators, and observe whether the patient has any adverse reactions.
05 Patient monitoring and adverse reaction management after transfusion
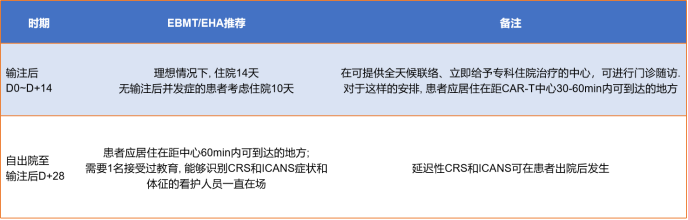
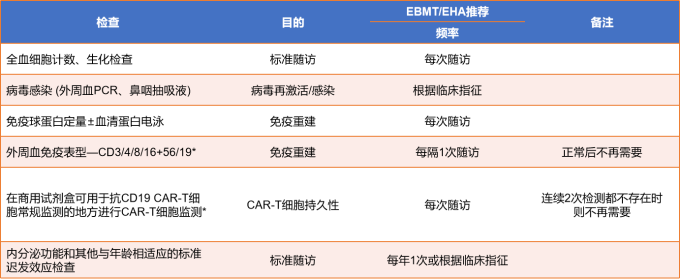
After the infusion, experts will monitor toxicity and side effects and uate efficacy. The monitoring will continue for 0-28 days after the infusion. The detection method is shown in Figure 9. The monitoring recommendations for 28-100 days after the infusion are shown in Figure 9.
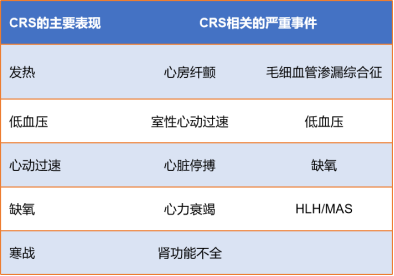
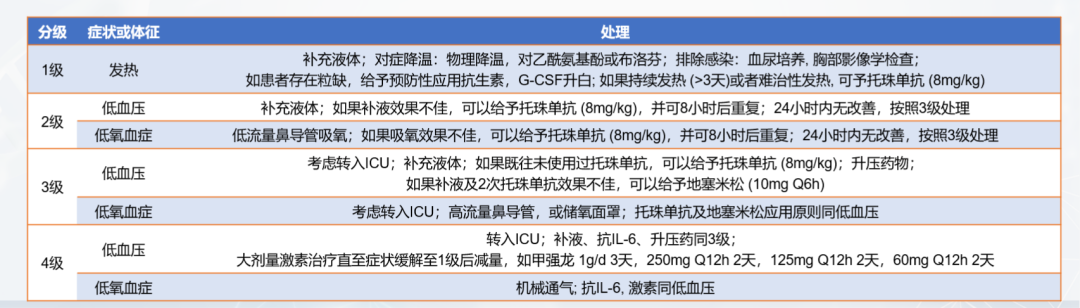
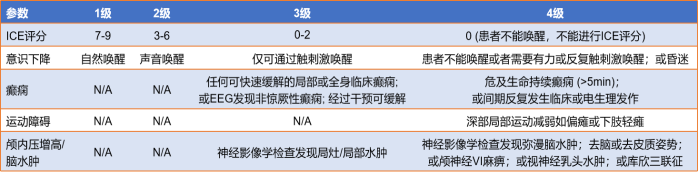
Cytokine release syndrome (CRS): It is a hyperphysiological reaction caused by the activation of endogenous or infused T cells and other immune cells in the body due to immunotherapy. IL-6/IL-6R is the main mediator of CRS. A large number of lymphocytes or bone marrow cells are activated and release inflammatory cytokines (such as IFN-γ, IL-6, TNF-α, IL-2), causing systemic SIRS, shock and vascular leakage. Common CRS is shown in Figure 10.

Cardiovascular complications : 10%-20% of CAR-T treated patients will experience them. Risk factors for CAR-T cardiotoxicity include ≥ grade 2 CRS, high disease burden, and cardiac dysfunction after previous exposure to cardiotoxins (including anthracyclines, radiotherapy, and tyrosine kinase inhibitors). Comprehensive cardiovascular uation before CAR-T cell infusion, appropriate monitoring, and risk reduction strategies can reduce CAR-T cardiovascular complications.
Tumor lysis syndrome (TLS): A group of syndromes caused by the massive disintegration of tumor cells, releasing cell contents and metabolites, including clinical manifestations such as hyperuricemia, hyperphosphatemia, hypocalcemia, hyperkalemia, and acute uric acid nephropathy.
06 Long-term follow-up after transfusion
Long-term follow-up should be performed by a multidisciplinary team (CAR-T physicians, disease-specific experts, long-term follow-up caregivers, data managers, clinical researchers) to capture disease status and late effects. Prolonged cytopenias, hypogammaglobulinemia, and infections are common; neurological complications and pulmonary toxicity increase the risk of death; secondary malignancies are rare.
Reference Leacture:
[1] Upadhaya S, et al. Nat Rev Drug Discov. 2021 Jul;20(7):503-504.
[2]Li X, et al. Cell Mol Immunol 2022; 19(1):122-124.
[3]Oken MM, et al. Am J Clin Oncol. 1982 Dec;5(6):649-55.
[4]應(yīng)志濤, 等. 北京大學(xué)腫瘤醫(yī)院嵌合抗原受體T細(xì)胞治療淋巴瘤全流程管理原則. 白血病·淋巴瘤 2021; 30(11):674-684.
[5]Hayden PJ, et al. Ann Oncol 2022; 33(3):259-275.
[6] Yakoub-Agha I, et al. Haematologica 2020; 105(2):297-316.
[7]NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Management of Immunotherapy-Related Toxicities. 2022 v1.
[8] Strati P, et al. Blood Adv. 2020 Aug 25;4(16):3943-3951.
[9] Lee DW, et al. Blood. 2014 Jul 10;124(2):188-95.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people understand the new developments in biomedicine.The content of this article is for information exchange only. This platform remains neutral with respect to the content, statements, and opinion judgments in the article, and does not represent the position and opinions of Shenzhen Cell Valley.The relevant information in this article should not be used for diagnosis or treatment, and cannot replace professional medical advice. Our website will not assume any responsibility.The final interpretation of the above statement belongs to our company’s website. This statement will apply to articles shared on our website at all times. Thank you for your cooperation! Copyright statement: The copyright of the article belongs to Shenzhen Cell Valley. Individuals are welcome to forward it to friends, media or Any unauthorized reproduction by the organization to other platforms will be regarded as infringement.If you need to reprint, please contact email: contact@sz-cell.com



